Wow it's been a long time since I updated this blog. There's a very good reason for that-- I had all these great plans about how I was going to update as all my results came in and give a real-time look at the progress of science. Well, I have done exactly that, because the progress of science has been next to nil, especially in the lab. Here's the status of my Funaria project as of this week:
Phenotypes: For 300 sporophytes of Funaria, I took pictures of the seta (stalk), the capsule, and the spores, and tallied various things about them. I then used various statistical tests to observe some things about my Funaria populations, with some interesting results. For example, I found that within F. hygrometrica, there is significant correlation between size and latitude- sporophytes furthest north (like Ithaca, NY and Brattleboro, VT) were much smaller than sporophytes furthest south (like Durham, NC and Catlett, VA). This latitude gradient would be the perfect thing to explore further with DNA approaches...
Lab Work: I've now spent three months trying to develop microsatellites for Funaria, to use as DNA fingerprinting and paternity tests. Using two different methods, I've come up with only four sites in the whole genome that have the small repeating regions, and I don't even know if those are variable enough to use. This presents a problem, as microsatellites are expensive to develop and I don't really have much funding. Despite the promising results from my phenotype work, it appears I have a decision to make about the future of my PhD work.
Meanwhile, this semester I have adopted the project left over from another graduate student, for a class that I am taking. Originally it was just going to be me tying together some loose ends on the project and using the class as an excuse to take care of it. The project involves peatmoss, or Sphagnum, specifically the two species that I helped collect in my very first experience with mosses. I still have pictures from that field trip up here. It is a group of species that has always interested me, but I didn't want to "step on the toes" of another grad student's work. Now that he is no longer with the lab, further exploration of this group could fall to me. But should I switch projects now, after a year dead set on Funaria? I have to weigh the pros and cons of each:
Funaria hygrometrica
Advantages: Weedy, easy to find, grows in discrete clumps for easy population sampling, grows well in the greenhouse, has a short generation time, bisexual gametophytes.
Disadvantages: Expensive genetic marker development has not gone well, strong possibility of uninteresting results, between species issues unclear.
Sphagnum macrophyllum
Advantages: Extremely well developed genetic markers, known populations, interesting questions already established, project funded by the lab. Between-species issues easily studied.
Disadvantages: Does not grow well in the greenhouse, crossing experiments unlikely, populations much more spread out, many are asexual.
Part of the difficulty of deciding between them is that their advantages seem complimentary-- where one is unfit, the other thrives! I struggled with the decision for a month, but some signs kept popping up: I kept failing to identify microsatellites in Funaria, and my class project with Sphagnum was going well and getting my advisor and other professors pretty excited. I, meanwhile, was feeling relieved to have real data to analyze without worrying about whether all the lab work would be successful. In the end, most of the major topics I am interested in will not change with Sphagnum: I can still study inbreeding, I can still study mating systems, and I have a better chance to study speciation than I did with Funaria. The reasons to switch kept piling up and I officially made up my mind on Friday morning.
Of course, because these things always happen that way, on Friday afternoon I received an e-mail. It was from Sigma Xi, the research organization, notifying me that I would be awarded a Grant in Research. The proposal I had written months ago concerned funding to develop microsatellites for Funaria. I now have the funding for the project I convinced myself I no longer want to do! Luckily, it shouldn't be a problem for Sigma Xi if I use the money to study Sphagnum instead. Still, the irony is palpable. In any event, I got my first graduate research grant!
Look forward in the coming weeks for a general Restart of Gametophyte Junction. Now that I'm more clear about which project I want to do, I can give lots of background and details!
Sunday, December 14, 2008
Thursday, June 26, 2008
Interlude: Moss Poetry
This just came in to my mailbox from BryoNet, the mailing list for the world's bryologists. It comes from Brent Mishler, who is a top bryologist at the University of California, Berkeley (formerly at Duke!). He sends along this poem on mosses, from nineteenth century Britishman William Gardiner. A clockmaker by trade, Gardiner was an avid collector of mosses and published a few of the first several important books on mosses. This poem appeared in one of them, Twenty Lessons on British Mosses, first published in 1846.
I think I can safely say this is the greatest moss poem ever written.
O! Let us love the silken moss
That clothes the time-worn wall
For great its Mighty Author is,
Although the plant be small.
The God who made the glorious sun
That shines so clear and bright,
And silver moon, and sparkling stars,
That gem the brow of night-
Did also give the sweet green moss
Its little form so fair;
And, though so tiny in all its parts,
Is not beneath His care.
When wandering in the fragrant wood,
Where pale primroses grow
To hear the tender ring-dove coo,
And happy small birds sing,
We tread a fresh and downy floor,
By soft green mosses made ;
And, when we rest by woodland stream,
Our couch with them is spread.
In valley deep, on mountain high-
The mosses still are there :
The dear delightful little things-
We meet them everywhere!
And when we mark them in our walks,
So beautiful, though small,
Our grateful hearts should glow with love
To Him who made them all.
I think I can safely say this is the greatest moss poem ever written.
Wednesday, June 18, 2008
Mr Johnson, I'm ready for my closeup...
Things have been fairly busy for me in the lab the last couple of weeks. I haven't been too successful with my molecular work. While that is pretty vital for my thesis, I've kept my spirits up by setting up a workflow for myself measuring phenotypes. My whole approach is aimed at integrating molecular data (genotype) with the size and shape of an organism (phenotype). Folks working on flowering plants have a lot more things to measure than I do, so I have to get the best out of my measurements. I'm intending on measuring seta size, capsule size, capsule shape, and spore number.
The last of these, spore number, is truly the most relevant to an evolutionary biologist. In terms of evolution, the most "fit" individuals are the ones who produce the most offspring. So by measuring the number of spores produced in a capsule, that's a pretty good idea of how many offspring that capsule is going to have. Why bother measuring anything else? Well, besides "my advisor says so," it may be that other characteristics about a sporophyte are more indicative of an inbred individual. For example, inbred Funaria may produce the same number of spores, but from a shorter seta- they may not be able to disperse as far, or they may get blocked out by taller sporophytes. Inbred capsules themselves may be smaller (even with the same number of spores), which may mean that per spore, an inbred capsule gets less nutrition.
I have been puzzling over how to measure such small organisms since I got back from my field trip. I met with various professors, including an expert on morphometrics (the science of measuring things). He suggested a program called ImageJ, software available for free and developed by the NIH. The software is pretty amazing. Here's my process:
1) Take a picture of the whole sporophyte, removed from the gametophyte. Here's an individual of Funaria flavicans, from Mechanicsville, NY:

2) Import the image into ImageJ. I have to calibrate the measurements using the milimeter scale in the bottom right, and then I seperate the image in to seta and capsule in 16-bit grayscale:

3) I "threshhold" the image, by telling the program what's an "object" and what's "not object" based on whether a particular pixel has any information in it. This effectively turns the image into a binary "object/not object" image:
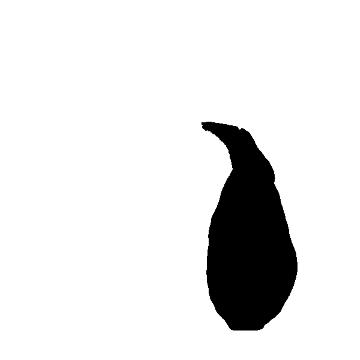
4) I use the "magic wand," familiar to anyone who's used a bit of image software before, to automatically draw a line hugging the outline of the binary image. I use the "Measure" command to automatically get the area inside the line, and the perimeter!
Taking the pictures using our microscope camera is the most time-consuming part. Once I have all the images it takes about half an hour to process twenty of them. The spore-counts will be a little different, and when I get started on them I'll tell you all how!
The last of these, spore number, is truly the most relevant to an evolutionary biologist. In terms of evolution, the most "fit" individuals are the ones who produce the most offspring. So by measuring the number of spores produced in a capsule, that's a pretty good idea of how many offspring that capsule is going to have. Why bother measuring anything else? Well, besides "my advisor says so," it may be that other characteristics about a sporophyte are more indicative of an inbred individual. For example, inbred Funaria may produce the same number of spores, but from a shorter seta- they may not be able to disperse as far, or they may get blocked out by taller sporophytes. Inbred capsules themselves may be smaller (even with the same number of spores), which may mean that per spore, an inbred capsule gets less nutrition.
I have been puzzling over how to measure such small organisms since I got back from my field trip. I met with various professors, including an expert on morphometrics (the science of measuring things). He suggested a program called ImageJ, software available for free and developed by the NIH. The software is pretty amazing. Here's my process:
1) Take a picture of the whole sporophyte, removed from the gametophyte. Here's an individual of Funaria flavicans, from Mechanicsville, NY:

2) Import the image into ImageJ. I have to calibrate the measurements using the milimeter scale in the bottom right, and then I seperate the image in to seta and capsule in 16-bit grayscale:

3) I "threshhold" the image, by telling the program what's an "object" and what's "not object" based on whether a particular pixel has any information in it. This effectively turns the image into a binary "object/not object" image:

4) I use the "magic wand," familiar to anyone who's used a bit of image software before, to automatically draw a line hugging the outline of the binary image. I use the "Measure" command to automatically get the area inside the line, and the perimeter!
Taking the pictures using our microscope camera is the most time-consuming part. Once I have all the images it takes about half an hour to process twenty of them. The spore-counts will be a little different, and when I get started on them I'll tell you all how!
Thursday, May 29, 2008
Nerd Beacon #2
Hey everyone. It's been a couple weeks since my last post, I've been hard at work trying to do multiple things to get my research going:
1. Find variable microsatellites so I can conduct population genetics studies.
2. Document my various collecting sites (also known as localities) using GPS and Google Earth, so I can have exact distances between collections.
3. Begin to measure traits (phenotypes) of the Funaria I collected, because the goal is to correlate relatedness with these traits.
I've been having trouble with all three, unfortunately. For the microsats, I've been having glitches with the equipment and my materials. One of our PCR machines broke while my experiment was running, and so that got ruined. I also am having trouble finding a program that lets me do all the sophisticated distance analysis I want that is also free. Finally, measuring traits is a bit difficult due to the shapes of the organisms.
In an evolutionary sense, the "fitness" of an organism is based upon how many offspring it has. For Funaria, the obvious thing to measure, then, is the number of spores each organism produces. However, this is a pretty time-consuming operation. Since each sporophyte produces 500,000 spores, I'm hoping I can use some other measure as a proxy for spore counts. One option is to measure the size of the capsule. However, this can be a pain because the capsule of Funaria is so oblique, rounded, and asymmetric. I'm meeting with a professor who specializes in measuring organisms tomorrow, so I hope he can help!
In the meantime, my only option is to measure the spores. The way I do this is by carefully rupturing a sporophyte into a small test tube that contains exactly one milliliter (mL) of sterile water. I then remove 1/10th of that (1 microliter) and place it on a special microscope slide known as a hemocytometer. The grids on that slide will allow me to count a small number of spores and then extrapolate to the whole sample. To count, I use what I consider to be nerd beacon #2- the clicker-counter:

This little device makes me happy on a number of levels- it keeps track of how many spores I see, sure. But more importantly, it is frequently used in baseball to keep track of pitch counts. Any combination of biology and baseball is definitely one I cherish.
1. Find variable microsatellites so I can conduct population genetics studies.
2. Document my various collecting sites (also known as localities) using GPS and Google Earth, so I can have exact distances between collections.
3. Begin to measure traits (phenotypes) of the Funaria I collected, because the goal is to correlate relatedness with these traits.
I've been having trouble with all three, unfortunately. For the microsats, I've been having glitches with the equipment and my materials. One of our PCR machines broke while my experiment was running, and so that got ruined. I also am having trouble finding a program that lets me do all the sophisticated distance analysis I want that is also free. Finally, measuring traits is a bit difficult due to the shapes of the organisms.
In an evolutionary sense, the "fitness" of an organism is based upon how many offspring it has. For Funaria, the obvious thing to measure, then, is the number of spores each organism produces. However, this is a pretty time-consuming operation. Since each sporophyte produces 500,000 spores, I'm hoping I can use some other measure as a proxy for spore counts. One option is to measure the size of the capsule. However, this can be a pain because the capsule of Funaria is so oblique, rounded, and asymmetric. I'm meeting with a professor who specializes in measuring organisms tomorrow, so I hope he can help!
In the meantime, my only option is to measure the spores. The way I do this is by carefully rupturing a sporophyte into a small test tube that contains exactly one milliliter (mL) of sterile water. I then remove 1/10th of that (1 microliter) and place it on a special microscope slide known as a hemocytometer. The grids on that slide will allow me to count a small number of spores and then extrapolate to the whole sample. To count, I use what I consider to be nerd beacon #2- the clicker-counter:
This little device makes me happy on a number of levels- it keeps track of how many spores I see, sure. But more importantly, it is frequently used in baseball to keep track of pitch counts. Any combination of biology and baseball is definitely one I cherish.
Thursday, May 15, 2008
Definition Train: Microsatellites
Next up stop for the definition train is not a concept but a technique, and one essential to my work. Microsatellites are also known as Short Tandem Repeats, and are DNA regions that contain a lot of repetitive DNA. Because they are very variable within a population, they're known as a "DNA Fingerprinting" technique, and are used for all kinds of purposes including forensics (they're featured on CSI quite often). Here's a little bit about how they are designed, and how they work.
Our DNA contains a lot of junk. That statement is less true than when it was first realized that despite the six billion base pairs of the human genome, less than a tenth of that is actually used for constructing proteins. Some of the rest of the genome is used to regulate gene expression, while much of it remains junk. However, junk is good for population geneticists, because junk DNA is not usually subject to natural selection; there aren't any constraints keeping a particular DNA sequence intact. They are free to vary according to the rules of chance, and those rules allow scientists to deduce how related two organisms are.
One type of these DNA regions contains repetitive bases- remember, the DNA code has four letters (A, G, C, and T). In these regions, the same two or three letters repeat many times. For example, it may look like this:
GATCAGTAAGATATATATATATATATATATATATATATATGGGTGCTCAGAT
In this case, this individual has the microsatellite "AT" repeated 16 times. The number of repeats turns out to be pretty variable, even within a population: one individual may have 16 repeats, as above, while a neighbor may have 12 repeats or 20 repeats. The interesting thing is that there are many of these regions (also known as loci) in the genome, and so one can have a pretty complete profile of an individual by looking at 5 to 15 of these loci:
Microsat Individual 1 Individual 2
1.............. 16................ 18
2............. 200 ............. 225
3.............. 75 ............... 75
4 ............. 87................ 90
5............ 103............... 115
This is a typical result of a microsat analysis; a machine reads off how many copies there are for each sample at each locus. With five loci, we can see that even though the two individuals are identical at locus 3, they are clearly two different individuals. Perhaps they are related, though. In forensics, crime labs will typically look at 13 loci to make a match between a suspect and a DNA sample from the crime scene. You might also hear on CSI or Court TV when it's said that the suspect has "7 of 13 alleles in common." What they mean is that at 7 of the 13 loci, two samples have identical numbers of repeats. This is extremely unlikely to be due to chance, and so it is pretty likely that the two samples come from DNA of blood-relatives (probably siblngs or parent-offspring).
It is one of my goals to investigate the matting patterns in mosses, by knowing who mates with whom. To do this, I need to find the regions (or loci) that have the microsatellites. Doing that from scratch is actually a time (3-4 months) and cost ($6000) intensive process. I'm fortunate that Funaria is in the same family as Physcomitrella, the lab rat of the moss world. Physcomitrella recently has its entire genome sequenced, and has a lot of researchers working on it, including those who have designed microsattelites for the species. In a paper, they also noted that many of the regions for Physcomitrella also work for Funaria. Those scientists have graciously supplied me with materials to discover whether those regions are variable enough for me to distinguish two moss individuals as they do with humans on CSI.
In addition, I hope to be able to tell whether the two species of Funaria (F. hygrometrica and F. flavicans) are hybridizing in nature. I would do this by examining populations where they are sympatric, and comparing them to populations which are allopatric. If the microsatellites show that the two species are more similar (by having more similar microsatellite profiles) when they are in sympatry than when in allopatry, it is likely that they are sharing genes.
Our DNA contains a lot of junk. That statement is less true than when it was first realized that despite the six billion base pairs of the human genome, less than a tenth of that is actually used for constructing proteins. Some of the rest of the genome is used to regulate gene expression, while much of it remains junk. However, junk is good for population geneticists, because junk DNA is not usually subject to natural selection; there aren't any constraints keeping a particular DNA sequence intact. They are free to vary according to the rules of chance, and those rules allow scientists to deduce how related two organisms are.
One type of these DNA regions contains repetitive bases- remember, the DNA code has four letters (A, G, C, and T). In these regions, the same two or three letters repeat many times. For example, it may look like this:
GATCAGTAAGATATATATATATATATATATATATATATATGGGTGCTCAGAT
In this case, this individual has the microsatellite "AT" repeated 16 times. The number of repeats turns out to be pretty variable, even within a population: one individual may have 16 repeats, as above, while a neighbor may have 12 repeats or 20 repeats. The interesting thing is that there are many of these regions (also known as loci) in the genome, and so one can have a pretty complete profile of an individual by looking at 5 to 15 of these loci:
Microsat Individual 1 Individual 2
1.............. 16................ 18
2............. 200 ............. 225
3.............. 75 ............... 75
4 ............. 87................ 90
5............ 103............... 115
This is a typical result of a microsat analysis; a machine reads off how many copies there are for each sample at each locus. With five loci, we can see that even though the two individuals are identical at locus 3, they are clearly two different individuals. Perhaps they are related, though. In forensics, crime labs will typically look at 13 loci to make a match between a suspect and a DNA sample from the crime scene. You might also hear on CSI or Court TV when it's said that the suspect has "7 of 13 alleles in common." What they mean is that at 7 of the 13 loci, two samples have identical numbers of repeats. This is extremely unlikely to be due to chance, and so it is pretty likely that the two samples come from DNA of blood-relatives (probably siblngs or parent-offspring).
It is one of my goals to investigate the matting patterns in mosses, by knowing who mates with whom. To do this, I need to find the regions (or loci) that have the microsatellites. Doing that from scratch is actually a time (3-4 months) and cost ($6000) intensive process. I'm fortunate that Funaria is in the same family as Physcomitrella, the lab rat of the moss world. Physcomitrella recently has its entire genome sequenced, and has a lot of researchers working on it, including those who have designed microsattelites for the species. In a paper, they also noted that many of the regions for Physcomitrella also work for Funaria. Those scientists have graciously supplied me with materials to discover whether those regions are variable enough for me to distinguish two moss individuals as they do with humans on CSI.
In addition, I hope to be able to tell whether the two species of Funaria (F. hygrometrica and F. flavicans) are hybridizing in nature. I would do this by examining populations where they are sympatric, and comparing them to populations which are allopatric. If the microsatellites show that the two species are more similar (by having more similar microsatellite profiles) when they are in sympatry than when in allopatry, it is likely that they are sharing genes.
Monday, May 12, 2008
Definition Train: Sympatry
It is my ongoing goal to explain, in common terms, what it is that I'm doing with this half-decade in graduate school. To that end, I'm hoping to make everyone familiar with certain terms; once you understand them, they're no longer over-your-head jargon! So every so often at the Gametophyte Junction we'll hop on the definition train. First up: sympatry.
Sympatry, like many biology terms, is a word derived from Greek: sym/syn- (meaning same), -patry (meaning fatherland). Two species are therefore sym-patric when they share the same fatherland; they have ranges which overlap. The opposite of sympatry is allopatry, meaning different-fatherland, and describes two species which have non-overlapping ranges. In between is parapatric, meaning adjacent-fatherland, and implying that the ranges of two species share a border (like two countries), or sometimes they have a narrow overlap range.
The concept of sympatry is important because of gene flow and speciation (the formation of new species). Initially, new species start out as populations, freely exchanging genes throughout the generations. The principles of population genetics have taught us that when a population has free gene flow, it is very tough to have different varieties. When a new variety emerges, three things usually happen: 1) The new variety is more adapted to the environment, and spreads quickly, wiping out the older varieties. 2) The new variety is less adapted to the environment, and vanishes quickly. 3) The new variety is neutral with respect to how adapted to the environment it is, and these varieties fluctuate until they are eventually lost or adopted by the whole population.
Species, meanwhile, under the Biological Species Concept, are not just observed by their separate and discontinuous variety. To achieve this discontinuity, there must be some barrier to gene flow between the population with the new variety and the other populations. Typically, this is done in allopatry- some geographic barrier (like a mountain or a body of water) separates two populations for an extended period of time. During this time, the two populations adapt to environments that are almost certainly different. These different adaptations may also change the organism in such a way that if the barrier were removed (a gap in the mountain, land bridge over the body of water), the two populations may have trouble mating. This creates the barrier to gene flow.
You'll notice that when the barrier is removed, the two populations are now in sympatry. It is usually at this point that species are truly formed- when two populations have reduced gene flow among them in sympatry. On a practical level, this manifests usually as "hybrid breakdown." Most people are familiar with the mule- it is an offspring of a horse and a donkey, but the mule itself is sterile. Because the horse and donkey adapted to different environments, they evolved genes that are slightly incompatible; enough to create a live child but one that is sterile. Having sterile offspring is definitely a barrier to gene flow. It is also a waste of resources, and natural selection typically leads such populations to methods of avoiding creating these bad hybrids. Animals recognize members of their own species through sight and sound; plants change pollinators or chemicals on the surfaces of gametes in an effort to only use precious reproduction resources on making good children.
What does this have to do with me? Well, by and large most of the work on mosses has not been under the Biological Species Concept. Bryologists over the years have been guided by the principles of taxonomy, grouping organisms in to species and genera and families based on similar characteristics. Whether two moss species have any barriers to gene flow is largely unknown. So it is entirely possible that the two species I'm studying, Funaria hygrometrica and Funaria flavicans, actually exchange genes. If so, then they are, in my opinion, one species. I'm bringing this up in part because of the following picture:

This was from my field trip, taken near Dillwyn, Virginia. The green capsules are F. hygrometrica and the smaller red capsules are F. flavicans. They are growing right next to each other, sympatrically. This is not a unique finding, my advisor wrote a paper in 1992 describing a mine site which had both species and what appeared to be intermediates. However, no DNA work was done to determine if there were hybrid plants. Some of the questions that intrigue me include:
Do the two species hybridize? If not, what barrier to gene flow separates them?
If hybrids form, are they less "fit" than their parents?
What genes control the separation of the species? Are there many genes or just a few?
For example, the F. flavicans in the picture above are red because they matured earlier than F. hygrometrica. Timing in plant matings are very important; if hybrids between the species were to mature at some intermediate time, they may not find many mates. That would make them less "fit" because "number of offspring" is the definition of fitness.
That's all for now; as always, feel free to ask questions if there's something unclear. I'm always willing to find new ways to explain things!
Sympatry, like many biology terms, is a word derived from Greek: sym/syn- (meaning same), -patry (meaning fatherland). Two species are therefore sym-patric when they share the same fatherland; they have ranges which overlap. The opposite of sympatry is allopatry, meaning different-fatherland, and describes two species which have non-overlapping ranges. In between is parapatric, meaning adjacent-fatherland, and implying that the ranges of two species share a border (like two countries), or sometimes they have a narrow overlap range.
The concept of sympatry is important because of gene flow and speciation (the formation of new species). Initially, new species start out as populations, freely exchanging genes throughout the generations. The principles of population genetics have taught us that when a population has free gene flow, it is very tough to have different varieties. When a new variety emerges, three things usually happen: 1) The new variety is more adapted to the environment, and spreads quickly, wiping out the older varieties. 2) The new variety is less adapted to the environment, and vanishes quickly. 3) The new variety is neutral with respect to how adapted to the environment it is, and these varieties fluctuate until they are eventually lost or adopted by the whole population.
Species, meanwhile, under the Biological Species Concept, are not just observed by their separate and discontinuous variety. To achieve this discontinuity, there must be some barrier to gene flow between the population with the new variety and the other populations. Typically, this is done in allopatry- some geographic barrier (like a mountain or a body of water) separates two populations for an extended period of time. During this time, the two populations adapt to environments that are almost certainly different. These different adaptations may also change the organism in such a way that if the barrier were removed (a gap in the mountain, land bridge over the body of water), the two populations may have trouble mating. This creates the barrier to gene flow.
You'll notice that when the barrier is removed, the two populations are now in sympatry. It is usually at this point that species are truly formed- when two populations have reduced gene flow among them in sympatry. On a practical level, this manifests usually as "hybrid breakdown." Most people are familiar with the mule- it is an offspring of a horse and a donkey, but the mule itself is sterile. Because the horse and donkey adapted to different environments, they evolved genes that are slightly incompatible; enough to create a live child but one that is sterile. Having sterile offspring is definitely a barrier to gene flow. It is also a waste of resources, and natural selection typically leads such populations to methods of avoiding creating these bad hybrids. Animals recognize members of their own species through sight and sound; plants change pollinators or chemicals on the surfaces of gametes in an effort to only use precious reproduction resources on making good children.
What does this have to do with me? Well, by and large most of the work on mosses has not been under the Biological Species Concept. Bryologists over the years have been guided by the principles of taxonomy, grouping organisms in to species and genera and families based on similar characteristics. Whether two moss species have any barriers to gene flow is largely unknown. So it is entirely possible that the two species I'm studying, Funaria hygrometrica and Funaria flavicans, actually exchange genes. If so, then they are, in my opinion, one species. I'm bringing this up in part because of the following picture:

This was from my field trip, taken near Dillwyn, Virginia. The green capsules are F. hygrometrica and the smaller red capsules are F. flavicans. They are growing right next to each other, sympatrically. This is not a unique finding, my advisor wrote a paper in 1992 describing a mine site which had both species and what appeared to be intermediates. However, no DNA work was done to determine if there were hybrid plants. Some of the questions that intrigue me include:
Do the two species hybridize? If not, what barrier to gene flow separates them?
If hybrids form, are they less "fit" than their parents?
What genes control the separation of the species? Are there many genes or just a few?
For example, the F. flavicans in the picture above are red because they matured earlier than F. hygrometrica. Timing in plant matings are very important; if hybrids between the species were to mature at some intermediate time, they may not find many mates. That would make them less "fit" because "number of offspring" is the definition of fitness.
That's all for now; as always, feel free to ask questions if there's something unclear. I'm always willing to find new ways to explain things!
Thursday, May 8, 2008
Days 6&7&9: Homeward Bound (with Pictures!)
So, it's been almost a week since my last post. I apologize for that, it has been a long busy week, and I've been splitting my time between the greenhouse and helping Rebecca find an apartment in Durham (she's working at the Duke Lemur Center next year!) I'd like to complete the series about my trip. I've also gotten my pictures uploaded, so I can finally share with you all some of the places I've been. Click HERE for the whole Google album!!!
Image embedding seems to not work very well, so I'm just going to link to pictures. Click on them for a thousand extra words. For starters, here's Funaria in all its glory.That's from Tompkins, County New York, near West Danby, in the really interesting population I talked about here.
We were able to collect two populations on Friday, one in Charlestown, Rhode Island and another in Plainfield, Connecticut. Oddly enough, they were both along railroad tracks parallel to side streets each named "Railroad Avenue." We didn't do too much else on Friday, although we did collect mosses from around Rhode Island and visited Beavertail Lighthouse, at the southern tip of Conanicut Island (in the sound between Providence and Newport). It's the third-oldest lighthouse in the US, according to the signs. Here's me at the lighthouse, trying not to be cold.
After making it home on Friday night we enjoyed an excellent meal of Carolina-style BBQ. Piers seemed to enjoy it despite his preference for Memphis-style, and I've been eating the leftovers back in Durham all week. On Saturday morning, Piers and I got up and traveled to the exotic location of... about a mile from our house, in the Wallkill River Wildlife Refuge. The Duke Herbarium has only six collections from Sussex, County NJ, and most of the collections in the New York Botanical Garden are pre-1950 or even pre-1900. So everything we collected was useful for bryologists in general. We went over to Vernon to the railroad tracks and found a quite extensive population of Funaria there.
There was also a very nice older man from the shops across the street, Dan Børstad. At first
he thought we were trash collectors, but after I explained what we were doing, he was still happy to talk to me. He was from Norway and said he had studied Botany when there, and I told him about all of our Norwegian colleagues who study Sphagnum up in the massive peat bogs. He also seemed impressed that I had returned all the way home just to collect the moss.
In the afternoon, Dad wanted to join us to see how the whole moss collecting operates. We were mostly searching for Sphagnum, which is the only moss that has been collected from the county. Most of the habitats we were looking at, however, looked more like this, which is far too eutrophic for Sphagnum.
We found a decent sized Funaria population (where I put Dad to work) in Sparta and hunted around for some of the previous localities for Sphagnum. One place we visited was Edison Pond, which I did not know existed. It's about 2 miles SSE of Ogdensburg, and was a series of mines funded by Thomas Edison in the early 1900s. He poured $2 million of his own money into the site to mine for iron, and employed 500 men at the peak of construction. There was no iron there, however, as the Hamburg Mountains are made almost entirely out of zinc and lime. The pond, and presumably the Sphagnum collected in 1986, were also gone.
Leaving Edison Pond, we headed up to High Point, and passed on entering the park itself to visit a swampy area at the base of Steenykill Lake, and found copious amounts of Sphagnum! Sadly, there weren't any sporophytes, but I'm hoping to come home again sometime in July, and perhaps I will be luckier then. The grad student working on them, however, is less convinced, and she thinks these species of Sphagnum never enter the sexual stage. How sad.
We settled in for an evening of Lasagana and a moss show-and-tell for my parents. The next morning we left, laden with moss and bagels from New Jersey, headed back to Durham. We stopped back in Bethesda for lunch, and then once in Catlett, Virginia to make a final Funaria collection.
It's going to be a lengthy job to sort out and document all the Funaria I collected during this trip. I had a lot of fun though, learning not just about the species I'm focusing on, but also gaining more field experience with mosses by traveling with such a knowledgeable guide. Even five days after returning, I'm thinking back upon what a whirlwind trip it was. I've already discovered some very intruiging things about the plants I collected this week; I hope to share some of those discoveries with you soon. So, by all means do not think that the end of my trip signals the end of my blogging- there's many more Fun(aria) to come!
Total Miles Traveled: 1,987
Total Funaria Populations: 18
Oil Pans Replaced: 1
Clichéd Advertising Reference: Priceless.
Image embedding seems to not work very well, so I'm just going to link to pictures. Click on them for a thousand extra words. For starters, here's Funaria in all its glory.That's from Tompkins, County New York, near West Danby, in the really interesting population I talked about here.
We were able to collect two populations on Friday, one in Charlestown, Rhode Island and another in Plainfield, Connecticut. Oddly enough, they were both along railroad tracks parallel to side streets each named "Railroad Avenue." We didn't do too much else on Friday, although we did collect mosses from around Rhode Island and visited Beavertail Lighthouse, at the southern tip of Conanicut Island (in the sound between Providence and Newport). It's the third-oldest lighthouse in the US, according to the signs. Here's me at the lighthouse, trying not to be cold.
After making it home on Friday night we enjoyed an excellent meal of Carolina-style BBQ. Piers seemed to enjoy it despite his preference for Memphis-style, and I've been eating the leftovers back in Durham all week. On Saturday morning, Piers and I got up and traveled to the exotic location of... about a mile from our house, in the Wallkill River Wildlife Refuge. The Duke Herbarium has only six collections from Sussex, County NJ, and most of the collections in the New York Botanical Garden are pre-1950 or even pre-1900. So everything we collected was useful for bryologists in general. We went over to Vernon to the railroad tracks and found a quite extensive population of Funaria there.
There was also a very nice older man from the shops across the street, Dan Børstad. At first
he thought we were trash collectors, but after I explained what we were doing, he was still happy to talk to me. He was from Norway and said he had studied Botany when there, and I told him about all of our Norwegian colleagues who study Sphagnum up in the massive peat bogs. He also seemed impressed that I had returned all the way home just to collect the moss.
In the afternoon, Dad wanted to join us to see how the whole moss collecting operates. We were mostly searching for Sphagnum, which is the only moss that has been collected from the county. Most of the habitats we were looking at, however, looked more like this, which is far too eutrophic for Sphagnum.
We found a decent sized Funaria population (where I put Dad to work) in Sparta and hunted around for some of the previous localities for Sphagnum. One place we visited was Edison Pond, which I did not know existed. It's about 2 miles SSE of Ogdensburg, and was a series of mines funded by Thomas Edison in the early 1900s. He poured $2 million of his own money into the site to mine for iron, and employed 500 men at the peak of construction. There was no iron there, however, as the Hamburg Mountains are made almost entirely out of zinc and lime. The pond, and presumably the Sphagnum collected in 1986, were also gone.
Leaving Edison Pond, we headed up to High Point, and passed on entering the park itself to visit a swampy area at the base of Steenykill Lake, and found copious amounts of Sphagnum! Sadly, there weren't any sporophytes, but I'm hoping to come home again sometime in July, and perhaps I will be luckier then. The grad student working on them, however, is less convinced, and she thinks these species of Sphagnum never enter the sexual stage. How sad.
We settled in for an evening of Lasagana and a moss show-and-tell for my parents. The next morning we left, laden with moss and bagels from New Jersey, headed back to Durham. We stopped back in Bethesda for lunch, and then once in Catlett, Virginia to make a final Funaria collection.
It's going to be a lengthy job to sort out and document all the Funaria I collected during this trip. I had a lot of fun though, learning not just about the species I'm focusing on, but also gaining more field experience with mosses by traveling with such a knowledgeable guide. Even five days after returning, I'm thinking back upon what a whirlwind trip it was. I've already discovered some very intruiging things about the plants I collected this week; I hope to share some of those discoveries with you soon. So, by all means do not think that the end of my trip signals the end of my blogging- there's many more Fun(aria) to come!
Total Miles Traveled: 1,987
Total Funaria Populations: 18
Oil Pans Replaced: 1
Clichéd Advertising Reference: Priceless.
Friday, May 2, 2008
Day 5: All the Live-Long Day
Inevitably, everything that is exciting will eventually become mundane. An event that was once a "Eureka!" moment leading to instant happiness becomes routine, ordinary, and relatively uninteresting. This is how I feel about collecting Funaria; now that we have our senses tuned into the habitat of the plant, finding it is as easy as finding railroad tracks. These we found about ten miles from where we stayed in Maltaville, and again right along the Connecticut River, at the border between Vermont and New Hampshire. Finding Funaria, even large populations, is now routine and not very exciting. I can go from seeing train tracks to being back in the car with 20 sample bags within like twenty minutes.
This underscores another importance of general moss collecting, that I talked about yesterday. It breaks up the monotony of only collecting one plant from essentially one habitat, allowing me to explore dense forests and mountain streams to collect whatever I find interesting. Piers usually ends up staying out of the car longer than I, but that's okay, because it allows me to write and to plan our route. The collections will continue to interrupt the monotony when I get back to Durham, as well- it's going to get boring, measuring seta lengths and doing DNA extractions. Identifying mosses, meanwhile, is like solving a puzzle. I work my way through a giant key to mosses, looking at microscopic characters that distinguish families, genera, and species.
We were almost completely stumped by the state of New Hampshire. At least in the southern part of the state, they have turned all the unused railways into hiking trails, removing the rails, ties, and gravel. I joked that Funaria should be listed as critically endangered in the state if they continue to remove its precious habitat. We were only successful in finding two patches of Funaria flavicans, but at least we didn't totally strike out.
Massachusetts also had its interesting moments. Driving south to Worcester (which I will never be able to pronounce correctly, according to Mike's friend Max), we passed over some railroad tracks. We pulled into a side street to investigate and found Funaria same as ever. As I was collecting, I noticed Piers was gone, which is not unusual, since he sometimes wanders off in search of something less weedy. What was unusual was the two state trooper cars sitting next to mine on the road. Apparently across the street from these tracks was a state corrections facility, and the troopers were not too happy I was there. Luckily, though, one of them was a Marine who spent a lot of time in Camp Lejeune, and so was a Duke fan. I shared some stories with him and he let us go without much problem. Oh well, it's not a true field trip unless you run into the authorities at least once.
We got to Providence and hit a bit of a snag as the guy we were staying with wasn't at his apartment. While we waited for him, we took out some bags from the very first stop, at that lumber yard in central Virginia. We hadn't seen anything like the samples we collected since, and it maybe that most of the population isn't Funaria at all! We won't know for sure until we get back to the lab. The good news is that I know at least some of the plants are Funaria. On top of that, what really mattered that day is that we THOUGHT it was Funaria, because at that point we were really frustrated by our Funaria-detecting ability. We're experts now, and we know what to do- follow the trains.
I got to stop by Brown and see Mike, which was very nice- we played some tennis (exercise thoroughly confused my body, which has begun to mesh with my drivers' seat), got some pizza, and met with some of his friends. Tomorrow, I'm headed home, to Hardyston New Jersey, via Rhode Island, Connecticut, and southern New York. Mom has promised pulled pork BBQ, which I'm sure will be up to Piers' Arkansas standards!
Miles Traveled: 255
Funaria Populations: 4
This underscores another importance of general moss collecting, that I talked about yesterday. It breaks up the monotony of only collecting one plant from essentially one habitat, allowing me to explore dense forests and mountain streams to collect whatever I find interesting. Piers usually ends up staying out of the car longer than I, but that's okay, because it allows me to write and to plan our route. The collections will continue to interrupt the monotony when I get back to Durham, as well- it's going to get boring, measuring seta lengths and doing DNA extractions. Identifying mosses, meanwhile, is like solving a puzzle. I work my way through a giant key to mosses, looking at microscopic characters that distinguish families, genera, and species.
We were almost completely stumped by the state of New Hampshire. At least in the southern part of the state, they have turned all the unused railways into hiking trails, removing the rails, ties, and gravel. I joked that Funaria should be listed as critically endangered in the state if they continue to remove its precious habitat. We were only successful in finding two patches of Funaria flavicans, but at least we didn't totally strike out.
Massachusetts also had its interesting moments. Driving south to Worcester (which I will never be able to pronounce correctly, according to Mike's friend Max), we passed over some railroad tracks. We pulled into a side street to investigate and found Funaria same as ever. As I was collecting, I noticed Piers was gone, which is not unusual, since he sometimes wanders off in search of something less weedy. What was unusual was the two state trooper cars sitting next to mine on the road. Apparently across the street from these tracks was a state corrections facility, and the troopers were not too happy I was there. Luckily, though, one of them was a Marine who spent a lot of time in Camp Lejeune, and so was a Duke fan. I shared some stories with him and he let us go without much problem. Oh well, it's not a true field trip unless you run into the authorities at least once.
We got to Providence and hit a bit of a snag as the guy we were staying with wasn't at his apartment. While we waited for him, we took out some bags from the very first stop, at that lumber yard in central Virginia. We hadn't seen anything like the samples we collected since, and it maybe that most of the population isn't Funaria at all! We won't know for sure until we get back to the lab. The good news is that I know at least some of the plants are Funaria. On top of that, what really mattered that day is that we THOUGHT it was Funaria, because at that point we were really frustrated by our Funaria-detecting ability. We're experts now, and we know what to do- follow the trains.
I got to stop by Brown and see Mike, which was very nice- we played some tennis (exercise thoroughly confused my body, which has begun to mesh with my drivers' seat), got some pizza, and met with some of his friends. Tomorrow, I'm headed home, to Hardyston New Jersey, via Rhode Island, Connecticut, and southern New York. Mom has promised pulled pork BBQ, which I'm sure will be up to Piers' Arkansas standards!
Miles Traveled: 255
Funaria Populations: 4
Thursday, May 1, 2008
Day 4: Misty Mountain Hop
Four years ago this month I had my first breakthrough in science- I was accepted into the NSF's Research Experience for Undergraduates program at Duke. The program was called "Bioinformatics and Systematics of Plants and Fungi." Many of the students were working on building phylogenies for the various groups of organisms they were interested in. I, on the other hand, was interested in speciation and just happened to be working on plants. The group went on a field trip to the Croatan National Forest in SE North Carolina and, walking around the savanna most of the other students were interested in all the different kinds of plants around. I was not, and I took some pretty pictures of flowers and trees, but I said to myself "I could never be as interested in plants the way those guys are. I just want to know about evolution."
Flash forward to April 30, 2008- a day I spent in the southern Adirondack Mountains doing nothing other than collecting as many different types of mosses- (mosses!) as I could find. 2004Matt would have slapped 2008Matt upside the head, "what the hell you wastin your time doin that for?!?" But he'd be wrong. What 2004Matt didn't understand is that you do need to care about the organisms in order to know about evolution. It's a pratical matter, as well: if I'm interested in a certain species, even if it's a model organism worked on almost entirely in the laboratory, I still need to collect specimens. In order to do that, I need to know how to find the species, and when I do collect it, I need to know what information to record about a site. So I need to know a lot about the ecology of the species, common associated other organisms, and a lot about its life cycle just to collect the organism.
So it's not enough to just claim that it doesn't matter what species I work on. While it's true that there are certain principles of evolution and genetics which broadly apply to all things (they do, after all, have DNA and are subject to natural selection). Still, it is the very qualities that make organisms unique which need to be accounted for during the whole process. I fear that some of my colleagues forget that, and I am very happy to have a serious field component of my research. Working on a group of organisms like mosses really lets me appreciate the diversity of life in new ways, in areas of forests that are usually trampled upon without thought.
To me, a day like I had in the Adirondacks was just as important as all the stops collecting Funaria. Yes, I'm going to be working on a particular species of moss, but it is still essential to understand the variation within the moss world, as each species has a unique method of living and reproducing. Getting myself locked in to one species would be a shame, so I didn't mind that we didn't collect Funaria at all in the Adirondacks. We didn't really look for it either, we just tried to collect interesting mosses in every NY county we passed on our way to Maltaville, NY at the end of the day.
When we got to the place we were staying, there were two other travelers staying there as well, a pair of men from Denmark, who are traveling all over the eastern US from March until June. Their purpose is to listen to Americans from all walks of life to get a sense of our culture. They're writing about it for their hometown newspaper and hope to get a book out of it. They also have a website, and I'm mentioned in there somewhere...
The next day is a whirlwind tour of three New England states: Vermont, New Hampshire, and Massachusetts, on our way to Providence, RI. I'm looking forward to meeting up with my brother, who goes to Brown, and Piers is looking forward to collecting in Rhode Island: the only eastern US state missing from his personal collection.
Miles Traveled: 243
Funaria populations: 0 (by design, but plenty of mosses collected!)
Flash forward to April 30, 2008- a day I spent in the southern Adirondack Mountains doing nothing other than collecting as many different types of mosses- (mosses!) as I could find. 2004Matt would have slapped 2008Matt upside the head, "what the hell you wastin your time doin that for?!?" But he'd be wrong. What 2004Matt didn't understand is that you do need to care about the organisms in order to know about evolution. It's a pratical matter, as well: if I'm interested in a certain species, even if it's a model organism worked on almost entirely in the laboratory, I still need to collect specimens. In order to do that, I need to know how to find the species, and when I do collect it, I need to know what information to record about a site. So I need to know a lot about the ecology of the species, common associated other organisms, and a lot about its life cycle just to collect the organism.
So it's not enough to just claim that it doesn't matter what species I work on. While it's true that there are certain principles of evolution and genetics which broadly apply to all things (they do, after all, have DNA and are subject to natural selection). Still, it is the very qualities that make organisms unique which need to be accounted for during the whole process. I fear that some of my colleagues forget that, and I am very happy to have a serious field component of my research. Working on a group of organisms like mosses really lets me appreciate the diversity of life in new ways, in areas of forests that are usually trampled upon without thought.
To me, a day like I had in the Adirondacks was just as important as all the stops collecting Funaria. Yes, I'm going to be working on a particular species of moss, but it is still essential to understand the variation within the moss world, as each species has a unique method of living and reproducing. Getting myself locked in to one species would be a shame, so I didn't mind that we didn't collect Funaria at all in the Adirondacks. We didn't really look for it either, we just tried to collect interesting mosses in every NY county we passed on our way to Maltaville, NY at the end of the day.
When we got to the place we were staying, there were two other travelers staying there as well, a pair of men from Denmark, who are traveling all over the eastern US from March until June. Their purpose is to listen to Americans from all walks of life to get a sense of our culture. They're writing about it for their hometown newspaper and hope to get a book out of it. They also have a website, and I'm mentioned in there somewhere...
The next day is a whirlwind tour of three New England states: Vermont, New Hampshire, and Massachusetts, on our way to Providence, RI. I'm looking forward to meeting up with my brother, who goes to Brown, and Piers is looking forward to collecting in Rhode Island: the only eastern US state missing from his personal collection.
Miles Traveled: 243
Funaria populations: 0 (by design, but plenty of mosses collected!)
Wednesday, April 30, 2008
Day 3: Highs and Lows
I woke up Tuesday morning ready for more moss- I even had a dream about moss, that I was tiny and lived in one of those Pleuridium capsules I talked about yesterday. After a hearty (and free) breakfast, I walked out to the car raring to go- the air was crisp, the sky was rain-free, and the smell of motor oil wafted across my nose. Um, what? There was a large, iridescent puddle beneath my car. Taking a peak underneath, it was clear what happened: somewhere on one of those dirt roads in East Penn, I had punctured my oil pan.
This was my payback for not renting an SUV for this trip, which probably would have cost about $400; oddly also the estimate for repairs. I was lucky that we were in Scranton, which has a VW dealership, though. They were able to squeeze me in during midday, but in the meantime I was trapped in Scranton. We did make the best of the situation, finding two populations of Funaria on opposite sides of town. After lunch at a disappointing pizza place (Donio's: home of chewy, greasy pizza), we headed back to the dealership. The estimate was actually high, which set up the rest of the day nicely.
On Day 2, we realized what kind of habitat we really needed to look for: railroad tracks. On our TOPO map, we identified the train tracks between Scranton and Ithaca, and we followed them dutifully, with amazing success. We followed the Susquahanna River north towards the New York border, and found moderately sized populations in all three counties between Scranton and the NY border. Piers and I were getting especially good at recognizing whether a particular railroad track would be promising, based on the kinds of rocks used as filler. As we entered New York, we found the tracks again north of Waverly, and driving along it, we could actually pick out where it was, based on the habitat and the bright red seta.
After Waverly, we were two counties away from Ithaca, and my advisor had given me some areas to check out near Cornell. Still, after a successful pickup in Chemung County, Piers suggested we stop just over the Tompkins County border (still about 15 miles south of Ithaca). We pulled up to the railroad tracks and Piers went left down the tracks towards a small stream, and found a few small patches. I went to the right, and... let's just say that I need to keep redefining what I mean by Mother Lode. The population was spread out over the same area as the one from Auburn, PA that I collected in the rain, about 500 feet along the tracks. This population was different- Funaria was in very large patches for this kind of moss- up to 3 feet across!
It also occured on both sides of the tracks, in an interesting habitat. On the far side of the tracks, Funaria was in smaller patches, further from the tracks than on the near side. There was also a lot of other types of moss: typical ones that occur on disturbed soil, and also a fern ally called Equisetem. A good ecologist would be able to make better hypotheses, but my guess is that Funaria is being out-competed on these soils by the other plants, and is forced further up the bank. I will be very interested to learn if the genetics of the patches with respect to their habitat. I actually collected from a third type of habitat at this location, down by the stream. Funaria doesn't usually occur in wet places, so this was an interesting find. We spent about 2 and a half hours (or, three beers in Piers Time) here, so we didn't have time to explore Ithaca that night.
We did, however, eat at an excellent Thai/Asian restaurant called Tamarind. I had the coconut soup with chicken, which I really want to learn how to make. We ended up in Ludlowville, NY, 10 miles north of Ithaca, in a really neat house. The room I stayed in was a hexagon with windows covering five of the
six walls. Needless to say, I didn't sleep much past dawn...
Miles Traveled: 150
Funaria populations: 6
Tomorrow: The southern Adirondack Mountains, ending in Maltaville, NY.
This was my payback for not renting an SUV for this trip, which probably would have cost about $400; oddly also the estimate for repairs. I was lucky that we were in Scranton, which has a VW dealership, though. They were able to squeeze me in during midday, but in the meantime I was trapped in Scranton. We did make the best of the situation, finding two populations of Funaria on opposite sides of town. After lunch at a disappointing pizza place (Donio's: home of chewy, greasy pizza), we headed back to the dealership. The estimate was actually high, which set up the rest of the day nicely.
On Day 2, we realized what kind of habitat we really needed to look for: railroad tracks. On our TOPO map, we identified the train tracks between Scranton and Ithaca, and we followed them dutifully, with amazing success. We followed the Susquahanna River north towards the New York border, and found moderately sized populations in all three counties between Scranton and the NY border. Piers and I were getting especially good at recognizing whether a particular railroad track would be promising, based on the kinds of rocks used as filler. As we entered New York, we found the tracks again north of Waverly, and driving along it, we could actually pick out where it was, based on the habitat and the bright red seta.
After Waverly, we were two counties away from Ithaca, and my advisor had given me some areas to check out near Cornell. Still, after a successful pickup in Chemung County, Piers suggested we stop just over the Tompkins County border (still about 15 miles south of Ithaca). We pulled up to the railroad tracks and Piers went left down the tracks towards a small stream, and found a few small patches. I went to the right, and... let's just say that I need to keep redefining what I mean by Mother Lode. The population was spread out over the same area as the one from Auburn, PA that I collected in the rain, about 500 feet along the tracks. This population was different- Funaria was in very large patches for this kind of moss- up to 3 feet across!
It also occured on both sides of the tracks, in an interesting habitat. On the far side of the tracks, Funaria was in smaller patches, further from the tracks than on the near side. There was also a lot of other types of moss: typical ones that occur on disturbed soil, and also a fern ally called Equisetem. A good ecologist would be able to make better hypotheses, but my guess is that Funaria is being out-competed on these soils by the other plants, and is forced further up the bank. I will be very interested to learn if the genetics of the patches with respect to their habitat. I actually collected from a third type of habitat at this location, down by the stream. Funaria doesn't usually occur in wet places, so this was an interesting find. We spent about 2 and a half hours (or, three beers in Piers Time) here, so we didn't have time to explore Ithaca that night.
We did, however, eat at an excellent Thai/Asian restaurant called Tamarind. I had the coconut soup with chicken, which I really want to learn how to make. We ended up in Ludlowville, NY, 10 miles north of Ithaca, in a really neat house. The room I stayed in was a hexagon with windows covering five of the
six walls. Needless to say, I didn't sleep much past dawn...
Miles Traveled: 150
Funaria populations: 6
Tomorrow: The southern Adirondack Mountains, ending in Maltaville, NY.
Monday, April 28, 2008
A note on Commenting
A few people have e-mailed me to ask how they can leave comments. I have left things open, so that people can leave comments with a Google account, or without. When I log out, I can choose to leave a comment as "Name/URL" which allows me to simply put my name (and a link to my favorite website) when I leave a comment.
There is still a "word verification" when you leave a comment; this is to keep spam down. Just type the word you see into the box when you leave your comment. If you have any more trouble, don't hesitate to shoot off another e-mail!
Happy posting!
There is still a "word verification" when you leave a comment; this is to keep spam down. Just type the word you see into the box when you leave your comment. If you have any more trouble, don't hesitate to shoot off another e-mail!
Happy posting!
Day 2: The Mother Lode
I knew very early the theme for my trip's second day: rain. It was raining when we left Bethesda, and drove (thankfully) against the traffic up to Fredericksburg, where we got back on our favorite US highway- US-15. It was a short drive up to Gettysburg, and we took the business route towards town to see what was there. Piers revealed that he double majored in biology and history, so he had interests in Gettysburg other than biology. We didn't have much time to site-see though, and headed to the Visitor's Center in the Military Park, in search of a good map. It was still under construction, and had a lot of gravelly trails headed through loose forests, so rather than get the map, we took a look around. By now the rain was pretty steady and I was soaked, so it didn't matter that I had to get a little wet to cross a small stream.
The only moss of interest I found, though, was Physcomitrium pyriforme, a moss that has haunted us over the first two days. It's a member of the same family as Funaria (the Funariaceae), and from a distance (say, standing over a patch), the sporophytes look similar. However, Physcomitrium has a rather distinct capsule when examined up close: it's pyriform, as the species name suggests. Funaria, meanwhile, has a much more elongated capsule, so much so that it sometimes bends over. Anyway, same family, similar look, similar habitats mean that we both frequently bent over to examine what was ultimately the wrong moss. It's a bummer, but it eventually yielded results- after about an hour Piers found a few small patches of F. flavicans.
Since we were both soaked, we were content on staying on the highway from Gettysburg to Harrisburg. Once there he suggested we stop at a railway; it seemed like the area was a loading site, as there were a lot of tractor trailers. There was also a lot of dirty, disturbed soil, which we poked around in for a while. The main mosses here were Bryum argenteum again (which I collected from on top of gravel on an abandoned trailer), and Ceratodon purpureus, another weedy moss commonly studied for population genetics. Stuart McDaniel, who graduated from the same lab as me in 2005, did his PhD research on the species. But it's not my species, and moved on across the Susquehanna River; I didn't find either Bagel Street, nor the hat company.
The plan was to get on PA-443, which hugs the base of the Appalachian Mountains in Pennsylvania. Right as we got on it, we passed over the same train tracks as our last stop. The only way down was a "No Outlet" street called "Cemetery Road." Pretty fitting, combining our two most common destinations; perhaps that would breed good luck. We drove down it as far as we could and walked out to the tracks. Funaria, everywhere! Along a 200 foot transect, I collected 13 different patches, on both sides of the tracks. When I was done, I put them in a bag marked: Funaria: Mother Lode. If only I knew how wrong I was.
We were content to put a good bit of distance between us and the Mother Lode population, mostly since I hadn't eaten lunch yet and it was still a steady, chilly rain. After we did stop, a decision was made to take a country road, PA-895, towards Palmerton, the coal-contaminated town a bit north of Allentown, PA. We thought it would be more direct, but it was a lot more windy, and had a lot more small towns scattered around. One of them was Auburn, PA, and as we left, we went under a train tressel. Piers suggested we stop and I followed him around the sparse, blackened forest unsuccessfully (moss-wise). We then scaled the steep up to the tracks and....
...Clearly, I did not know what Mother Lode meant. This population was huuuuuuge! Now, for a moss that might be tough to imagine, but we walked about 400 feet on both sides of the train tressel and found patches all over the place, ranging from small (2 cm wide) to large (20-30 cm). One thing I found very interesting is that the setae (plural of seta: remember it's the stalk that the capsule is on) were very variable. Some of them were quite short (2 cm) while others were quite long (10-15 cm) by moss standards. One question I have about this is whether it's due to genetics, or to environment. I will have to do experiments, growing different varieties in the same environment, to determine this. If it is genetic, I will be able to determine if there is a correlation between seta height and relatedness: generally, are inbred mosses characterized by short seta?
We spent over ninety minutes at this site, and I collected 58 patches on both sides of the tracks. By the time I had gotten all the bags laid out, I then needed to measure the distances. About then, we heard a rumbling.... and a small train whisked by, sending alot of my bags flying! I was initially very mad, but then I remembered that all the bags were numbered, and Piers had placed a stick near every population as he walked by, so it was easy to put the bags back home. They (and we) were soaked through when we got back to the car, where we quickly transcribed all the info and rebagged the mosses. It was a ridiculously successful day, and we still had two hours of light left!
It was now clear that railroad tracks were the way to go. We opened our trusty Pennsylvania gazeteer to follow the tracks... and as we approached Palmerton we went where the map said we should find a choo-choo, but there was nothing. Piers asked the postmaster of scenic East Penn, PA, and he said the tracks had been removed, but there was still a trail where they used to be. We went there and searched for a while, but only found a few small patches- I'm spoiled by Mother Lodes, for sure.
We called it a day without getting to Palmerton. I don't think I'll regret it, as I slave away under a microscope this summer, measuring the thousands of plants we collected today. Tomorrow's plan: Ithaca is Gorges! It's a short, 2-hour drive from Scranton to Ithaca but we'll be taking our time, snaking along US-11 which apparently runs along a railway, and then over to some sites near Cornell that Jon Shaw suggested. The forecast calls for a lot less rain, but also a lot less temperature. Sounds like a multi-layer day.
Miles Traveled: 200
Funaria Populations: 4
The only moss of interest I found, though, was Physcomitrium pyriforme, a moss that has haunted us over the first two days. It's a member of the same family as Funaria (the Funariaceae), and from a distance (say, standing over a patch), the sporophytes look similar. However, Physcomitrium has a rather distinct capsule when examined up close: it's pyriform, as the species name suggests. Funaria, meanwhile, has a much more elongated capsule, so much so that it sometimes bends over. Anyway, same family, similar look, similar habitats mean that we both frequently bent over to examine what was ultimately the wrong moss. It's a bummer, but it eventually yielded results- after about an hour Piers found a few small patches of F. flavicans.
Since we were both soaked, we were content on staying on the highway from Gettysburg to Harrisburg. Once there he suggested we stop at a railway; it seemed like the area was a loading site, as there were a lot of tractor trailers. There was also a lot of dirty, disturbed soil, which we poked around in for a while. The main mosses here were Bryum argenteum again (which I collected from on top of gravel on an abandoned trailer), and Ceratodon purpureus, another weedy moss commonly studied for population genetics. Stuart McDaniel, who graduated from the same lab as me in 2005, did his PhD research on the species. But it's not my species, and moved on across the Susquehanna River; I didn't find either Bagel Street, nor the hat company.
The plan was to get on PA-443, which hugs the base of the Appalachian Mountains in Pennsylvania. Right as we got on it, we passed over the same train tracks as our last stop. The only way down was a "No Outlet" street called "Cemetery Road." Pretty fitting, combining our two most common destinations; perhaps that would breed good luck. We drove down it as far as we could and walked out to the tracks. Funaria, everywhere! Along a 200 foot transect, I collected 13 different patches, on both sides of the tracks. When I was done, I put them in a bag marked: Funaria: Mother Lode. If only I knew how wrong I was.
We were content to put a good bit of distance between us and the Mother Lode population, mostly since I hadn't eaten lunch yet and it was still a steady, chilly rain. After we did stop, a decision was made to take a country road, PA-895, towards Palmerton, the coal-contaminated town a bit north of Allentown, PA. We thought it would be more direct, but it was a lot more windy, and had a lot more small towns scattered around. One of them was Auburn, PA, and as we left, we went under a train tressel. Piers suggested we stop and I followed him around the sparse, blackened forest unsuccessfully (moss-wise). We then scaled the steep up to the tracks and....
...Clearly, I did not know what Mother Lode meant. This population was huuuuuuge! Now, for a moss that might be tough to imagine, but we walked about 400 feet on both sides of the train tressel and found patches all over the place, ranging from small (2 cm wide) to large (20-30 cm). One thing I found very interesting is that the setae (plural of seta: remember it's the stalk that the capsule is on) were very variable. Some of them were quite short (2 cm) while others were quite long (10-15 cm) by moss standards. One question I have about this is whether it's due to genetics, or to environment. I will have to do experiments, growing different varieties in the same environment, to determine this. If it is genetic, I will be able to determine if there is a correlation between seta height and relatedness: generally, are inbred mosses characterized by short seta?
We spent over ninety minutes at this site, and I collected 58 patches on both sides of the tracks. By the time I had gotten all the bags laid out, I then needed to measure the distances. About then, we heard a rumbling.... and a small train whisked by, sending alot of my bags flying! I was initially very mad, but then I remembered that all the bags were numbered, and Piers had placed a stick near every population as he walked by, so it was easy to put the bags back home. They (and we) were soaked through when we got back to the car, where we quickly transcribed all the info and rebagged the mosses. It was a ridiculously successful day, and we still had two hours of light left!
It was now clear that railroad tracks were the way to go. We opened our trusty Pennsylvania gazeteer to follow the tracks... and as we approached Palmerton we went where the map said we should find a choo-choo, but there was nothing. Piers asked the postmaster of scenic East Penn, PA, and he said the tracks had been removed, but there was still a trail where they used to be. We went there and searched for a while, but only found a few small patches- I'm spoiled by Mother Lodes, for sure.
We called it a day without getting to Palmerton. I don't think I'll regret it, as I slave away under a microscope this summer, measuring the thousands of plants we collected today. Tomorrow's plan: Ithaca is Gorges! It's a short, 2-hour drive from Scranton to Ithaca but we'll be taking our time, snaking along US-11 which apparently runs along a railway, and then over to some sites near Cornell that Jon Shaw suggested. The forecast calls for a lot less rain, but also a lot less temperature. Sounds like a multi-layer day.
Miles Traveled: 200
Funaria Populations: 4
Day 1: "This is a weed, right???"
So, I have some bad news. I packed the wrong USB-cord, so I won't be able to include pictures in my updates. Don't worry though, I'm taking plenty!
Today, we started off from Durham at 8 AM and headed up US-15 from Oxford. The name of the game was: find some cemeteries! I admit, it is a little odd to be walking around a cemetery looking for moss, but they do seem to have a great assortment of mosses. Many cemeteries don't have very high grass, and near the headstones or on clear dirt are great places to find moss growing. I usually stuck to the outskirts of the cemeteries, letting Piers wander around among the graves. The only collection I made all day from one was next to a headstone marked only with a name; I assumed that it was just a placeholder.
We stopped along the John H. Kerr Reservoir near the border of North Carolina and Virginia. There was a state park on the NC side, and it looked really promising. Funaria is a first-colonizer of recently burned-over soils, and campgrounds are usually a good bet. This campground featured a bunch of 10'x10' squares populated by a single table (wooden sitting platforms and a stone top), along with a circular metal fire pit. Some of the squares had obviously not been populated for some time, as they had moss growing even on top of the stone table! However, there was no Funaria.
We moved on north, to the Occoneechee State Park, because Piers had previously collected Funaria flavicans near a parking lot. Rather than pay $4 for a day pass, we convinced the ranger that we just need to get a GPS point, and so we couldn't stay long. Sure enough, we parked next to the entrance to the aptly named "Mossy Creek Trail," and in the grass next to the gravelly lot there were four small patches of F. flavicans! "Well, you can't call the trip a bust anymore," said Piers, unfortunately foreshadowing the rest of the day.
We stopped at many forest trails and cemeteries on our way north through Virginia, including the one I mentioned earlier, where I collected Bryum argenteum, at a cemetery near Sheppards, Virginia. After a few more sites (unsuccessful for Funaria but Piers collected a lot), we stopped for lunch in Farmville, VA. I didn't see many farms, and I must admit I was feeling a bit frustrated at this point. I said to Piers, "We're sure this thing is a weed, right?" Of course, it is, but Piers responded equally sardonically, "well, that's the danger of being a specialist... generalists always find what they're looking for!"
North of Farmville we came across some railroad tracks and a lumber yard a few miles north, near the town of Dillwyn. I turned in to check it out, intending to look mostly at the railroad tracks. Driving closer to a large building we noticed a man sitting in a forklift staring at us, so we slowly backed out towards the main road. Before we went to check out the RR tracks, I looked at a grassy area that led up this large ramp, at the end of which was an odd looking contraption that probably filled passing trains with sand, with a dispenser dangling precariously over the tracks. This will probably make more sense when I have a picture. Anyway, beneath my feet were clearly stems of Funaria! I collected many patches of the moss up the ramp, over about 200 feet. It was exciting, and I wanted to find more quickly, but it was getting late and we had a lot of miles to cover before spending the night at Sam's in Bethesda.
We only stopped once more, at another cemetary in Fluvall County, VA, where I collected an interesting moss, Pleuridium. It occurs on clay just like Funaria flavicans often does, but rather than having sporophytes with a long seta (the stalk), Pleuridium has barely any seta at all. The result is that the capsules, which release the spores for the next generation, are immersed, and they can't go all that far. It would be interesting to study the population dynamics of this species. But, another thesis for another lifetime, I guess.
We got in at Sam's at 9 PM, and happily chowed down on some chicken parm subs, due to a rare fit of initiative in picking a place to eat without three hours dicussion.... Overall a pretty successful first day, since I don't want to burn out collecting tons of stuff early on! Tomorrow's plan: make our way back to US-15, and take that to Gettysburg and Harrisburg, before turning east towards the coal-smelting town of Palmerton, and a final resting place near Scranton, PA.
Miles Traveled: 315
Funaria Populations: 2
Today, we started off from Durham at 8 AM and headed up US-15 from Oxford. The name of the game was: find some cemeteries! I admit, it is a little odd to be walking around a cemetery looking for moss, but they do seem to have a great assortment of mosses. Many cemeteries don't have very high grass, and near the headstones or on clear dirt are great places to find moss growing. I usually stuck to the outskirts of the cemeteries, letting Piers wander around among the graves. The only collection I made all day from one was next to a headstone marked only with a name; I assumed that it was just a placeholder.
We stopped along the John H. Kerr Reservoir near the border of North Carolina and Virginia. There was a state park on the NC side, and it looked really promising. Funaria is a first-colonizer of recently burned-over soils, and campgrounds are usually a good bet. This campground featured a bunch of 10'x10' squares populated by a single table (wooden sitting platforms and a stone top), along with a circular metal fire pit. Some of the squares had obviously not been populated for some time, as they had moss growing even on top of the stone table! However, there was no Funaria.
We moved on north, to the Occoneechee State Park, because Piers had previously collected Funaria flavicans near a parking lot. Rather than pay $4 for a day pass, we convinced the ranger that we just need to get a GPS point, and so we couldn't stay long. Sure enough, we parked next to the entrance to the aptly named "Mossy Creek Trail," and in the grass next to the gravelly lot there were four small patches of F. flavicans! "Well, you can't call the trip a bust anymore," said Piers, unfortunately foreshadowing the rest of the day.
We stopped at many forest trails and cemeteries on our way north through Virginia, including the one I mentioned earlier, where I collected Bryum argenteum, at a cemetery near Sheppards, Virginia. After a few more sites (unsuccessful for Funaria but Piers collected a lot), we stopped for lunch in Farmville, VA. I didn't see many farms, and I must admit I was feeling a bit frustrated at this point. I said to Piers, "We're sure this thing is a weed, right?" Of course, it is, but Piers responded equally sardonically, "well, that's the danger of being a specialist... generalists always find what they're looking for!"
North of Farmville we came across some railroad tracks and a lumber yard a few miles north, near the town of Dillwyn. I turned in to check it out, intending to look mostly at the railroad tracks. Driving closer to a large building we noticed a man sitting in a forklift staring at us, so we slowly backed out towards the main road. Before we went to check out the RR tracks, I looked at a grassy area that led up this large ramp, at the end of which was an odd looking contraption that probably filled passing trains with sand, with a dispenser dangling precariously over the tracks. This will probably make more sense when I have a picture. Anyway, beneath my feet were clearly stems of Funaria! I collected many patches of the moss up the ramp, over about 200 feet. It was exciting, and I wanted to find more quickly, but it was getting late and we had a lot of miles to cover before spending the night at Sam's in Bethesda.
We only stopped once more, at another cemetary in Fluvall County, VA, where I collected an interesting moss, Pleuridium. It occurs on clay just like Funaria flavicans often does, but rather than having sporophytes with a long seta (the stalk), Pleuridium has barely any seta at all. The result is that the capsules, which release the spores for the next generation, are immersed, and they can't go all that far. It would be interesting to study the population dynamics of this species. But, another thesis for another lifetime, I guess.
We got in at Sam's at 9 PM, and happily chowed down on some chicken parm subs, due to a rare fit of initiative in picking a place to eat without three hours dicussion.... Overall a pretty successful first day, since I don't want to burn out collecting tons of stuff early on! Tomorrow's plan: make our way back to US-15, and take that to Gettysburg and Harrisburg, before turning east towards the coal-smelting town of Palmerton, and a final resting place near Scranton, PA.
Miles Traveled: 315
Funaria Populations: 2
Saturday, April 26, 2008
An Epic Field Trip
One main motivation for starting this blog was to post updates from my first research field trip of grad school. Originally this trip was planned for after the semester, in about Mid-May. However, my advisor and I realized that Funaria, the genus I'm looking for, matures a bit earlier than we had previously thought. If they fully mature before I get there, they'll release their spores and be useless to me. So the whole field trip was moved up to... next week. Starting tomorrow.
I'm looking for populations of Funaria across a wide range, like the Northeast US. There are two objectives:
1) To examine the genetic structure of populations- how related are populations from NY to populations in Virginia? Remember, we're talking mosses here, so you have to think on a really small scale- is there a lot of mixing across the Eastern US, or are populations isolated?
2) How variable are populations? Moss can increase in numbers in two ways: either through sexual reproduction and spores (which fly through the wind to land on new soil), or vegetative reproduction. The latter would produce a population that is, essentially, one clone. This question is interesting if you're inclined to think about philosophical questions such as: What is an individual.
The other objective on the trip is to collect two species of moss: Funaria hygrometrica, and Funaria flavicans. These species are related, and it is possible that they can hybridize. If I can collect populations where both species occur, I may be able to find evidence that genes have been exchanged between the species. If this is true, it would open up a whole set of questions for my thesis, regarding the field of speciation. Briefly, the principles of natural selection make predictions about how species form and how they remain distinct. For closely related species, there is often something preventing them from crossing, which typically results in unfit offspring.
Both species of Funaria grow in similar areas: disturbed, sunny soil. For example, the first population I sampled was right next to the Duke greenhouse:

It's right there near those piles of dirt. It's most "natural" habitat is in recently burned areas, but mostly during this trip I will be look for cemeteries, forest paths, and railroad tracks. When they are completely mature and ready to release their spores, they're actually pretty distinctive, having a bright orange color that stands out from the surrounding grass or other moss:

My guide will be Piers Majestyk, who is a post doc in the lab and an excellent field bryologist and taxonomist. He's real laid back and should be fun to travel with, and I'm looking forward to learning a lot from him. This trip is small potatoes for him, as later this year he's going to be gone for three months, collecting a tropical moss genus (Daltoniaceae) from: Ethiopia, India, Nepal, Philippines, and Hawaii. That's intense.
So, where am I going? Here's the preliminary plan:
Sunday night: Bethesda, MD
Monday night: Scranton, PA
Tuesday night: Ithaca, NY
Wednesday night: Saratoga Falls, NY
Thursday night: Providence, RI
Friday and Saturday night: Hardyston, NJ
Sunday night: Salisbury, MD
Of course, that's just the sleeping places (200 years from now, there may be historic sites: Matt Johnson slept here!). There are a few interesting places I'm targeting:
Day 1: Central Virginia. I'll be taking the long way to the DC area, up US-15 rather than I-95. This way, we'll be able to stop at sites that look promising- cemeteries, open fields, forests that look recently burned.
Day 2: Pennsylvania: We'll leave the DC area and return to US-15, which goes through Gettysburg on its way to Harrisburg. One place I'm looking forward to exploring is Palmerton, PA, which is further east near Allentown. Over 100 years ago, it was the site of a coal smelter, and half the mountain near Palmerton has very contaminated soil. The southern half of the mountain, meanwhile, is clean. If I can find Funaria on both sides, there could be different population dynamics depending on the contamination.
Day 3: Ithaca: My advisor spent a lot of time at Cornell and Ithaca College in the 1980s, and so he is familiar with the areas. He's suggested some filtration ponds near Cornell's campus, and some hills near Ithaca to explore.
Day 4: Southern Adirondack Mountains: This is probably my best chance to find Funaria in a "natural" environment, one that won't be too terribly disturbed by humans, which could make it a good comparison to other, more disturbed populations.
Day 5: Vermont, New Hampshire, Massachusetts: We'll end this day in Providence, and hopefully collect Funaria from at least once site in each state I pass through.
Day 6: Rhode Island, Connecticut: Most of this day will be catching up with friends/family in Providence, with some bryologizing on the side :-)
Day 7: Sussex County, NJ: I may be biased, but I'm hoping there's an interesting population or three here, so that I can have a good excuse to go home to "study." We'll be hitting up High Point State Park and Stokes State Forest, perhaps also Wawayanda.
Day 8: Central/Southern NJ or Delaware. This will depend on how successful we've been, but one plan would be to visit some small parks in New Jersey, as well as the Pine Barrens. Another plan would be to head down the middle of Delaware towards Saisbury, MD. I already have an extensive collection from near Middleton, DE, but further down the penninsula could be interesting as well.
Day 9: Eastern North Carolina. Piers says that some of the swamps/parks in northeast North Carolina have been undercollected. While there may not be Funaria, it will be good to end the trip on a high note for the Duke Herbarium.
And then, back to Durham. My aim is to write interesting things that happen on the trip, and when I have internet access, update frequently, so check back soon!
I'm looking for populations of Funaria across a wide range, like the Northeast US. There are two objectives:
1) To examine the genetic structure of populations- how related are populations from NY to populations in Virginia? Remember, we're talking mosses here, so you have to think on a really small scale- is there a lot of mixing across the Eastern US, or are populations isolated?
2) How variable are populations? Moss can increase in numbers in two ways: either through sexual reproduction and spores (which fly through the wind to land on new soil), or vegetative reproduction. The latter would produce a population that is, essentially, one clone. This question is interesting if you're inclined to think about philosophical questions such as: What is an individual.
The other objective on the trip is to collect two species of moss: Funaria hygrometrica, and Funaria flavicans. These species are related, and it is possible that they can hybridize. If I can collect populations where both species occur, I may be able to find evidence that genes have been exchanged between the species. If this is true, it would open up a whole set of questions for my thesis, regarding the field of speciation. Briefly, the principles of natural selection make predictions about how species form and how they remain distinct. For closely related species, there is often something preventing them from crossing, which typically results in unfit offspring.
Both species of Funaria grow in similar areas: disturbed, sunny soil. For example, the first population I sampled was right next to the Duke greenhouse:

It's right there near those piles of dirt. It's most "natural" habitat is in recently burned areas, but mostly during this trip I will be look for cemeteries, forest paths, and railroad tracks. When they are completely mature and ready to release their spores, they're actually pretty distinctive, having a bright orange color that stands out from the surrounding grass or other moss:

My guide will be Piers Majestyk, who is a post doc in the lab and an excellent field bryologist and taxonomist. He's real laid back and should be fun to travel with, and I'm looking forward to learning a lot from him. This trip is small potatoes for him, as later this year he's going to be gone for three months, collecting a tropical moss genus (Daltoniaceae) from: Ethiopia, India, Nepal, Philippines, and Hawaii. That's intense.
So, where am I going? Here's the preliminary plan:
Sunday night: Bethesda, MD
Monday night: Scranton, PA
Tuesday night: Ithaca, NY
Wednesday night: Saratoga Falls, NY
Thursday night: Providence, RI
Friday and Saturday night: Hardyston, NJ
Sunday night: Salisbury, MD
Of course, that's just the sleeping places (200 years from now, there may be historic sites: Matt Johnson slept here!). There are a few interesting places I'm targeting:
Day 1: Central Virginia. I'll be taking the long way to the DC area, up US-15 rather than I-95. This way, we'll be able to stop at sites that look promising- cemeteries, open fields, forests that look recently burned.
Day 2: Pennsylvania: We'll leave the DC area and return to US-15, which goes through Gettysburg on its way to Harrisburg. One place I'm looking forward to exploring is Palmerton, PA, which is further east near Allentown. Over 100 years ago, it was the site of a coal smelter, and half the mountain near Palmerton has very contaminated soil. The southern half of the mountain, meanwhile, is clean. If I can find Funaria on both sides, there could be different population dynamics depending on the contamination.
Day 3: Ithaca: My advisor spent a lot of time at Cornell and Ithaca College in the 1980s, and so he is familiar with the areas. He's suggested some filtration ponds near Cornell's campus, and some hills near Ithaca to explore.
Day 4: Southern Adirondack Mountains: This is probably my best chance to find Funaria in a "natural" environment, one that won't be too terribly disturbed by humans, which could make it a good comparison to other, more disturbed populations.
Day 5: Vermont, New Hampshire, Massachusetts: We'll end this day in Providence, and hopefully collect Funaria from at least once site in each state I pass through.
Day 6: Rhode Island, Connecticut: Most of this day will be catching up with friends/family in Providence, with some bryologizing on the side :-)
Day 7: Sussex County, NJ: I may be biased, but I'm hoping there's an interesting population or three here, so that I can have a good excuse to go home to "study." We'll be hitting up High Point State Park and Stokes State Forest, perhaps also Wawayanda.
Day 8: Central/Southern NJ or Delaware. This will depend on how successful we've been, but one plan would be to visit some small parks in New Jersey, as well as the Pine Barrens. Another plan would be to head down the middle of Delaware towards Saisbury, MD. I already have an extensive collection from near Middleton, DE, but further down the penninsula could be interesting as well.
Day 9: Eastern North Carolina. Piers says that some of the swamps/parks in northeast North Carolina have been undercollected. While there may not be Funaria, it will be good to end the trip on a high note for the Duke Herbarium.
And then, back to Durham. My aim is to write interesting things that happen on the trip, and when I have internet access, update frequently, so check back soon!
Accidental Bryology
Back in December, the first-year grad students felt bored and slightly lost as we sat in a banquet hall at Washington Duke Inn. It was the Biology Department Retreat, although it was a very loose interpretation of the word "retreat," since we were about 200 yards from campus. An idea was born that day, though, that the first-years should have a real retreat of our own, and we were granted some funding to go someplace special. Although I voted for the Duke Marine Lab, the winning place was the Mountain Lake Biological Station. I'm sure the knowledge that Dirty Dancing was filmed at the nearby Mountain Lake Hotel swayed a few of the female voters. It's located at the edge of the Appalachian mountains near Blacksburg, VA, and I must say I'm glad we chose the spot, the weather was great and we had the whole place to ourselves.
Since I'm taking a Bryology class this semester, I've gotten in the habit of taking my collecting bag wherever I go. It's complete with my GPS, Camera, small paper bags, and pens. It also contains a 14x hand lens, which others have lovingly referred to as a "nerd beacon," as you can always tell a naturalist from the small lens draped around his neck. Throughout the day, I kept looking for Funaria in its customary places: near old campfires, along the edges of the paths. Plenty of moss everywhere, but no Funaria. That was fine, and I had fun telling the laboratory-stricken among us all about bryophytes. Later in the day, there was a movement to hike up to Bear Cliffs, which is a sheer cliff-face that overlooks all of the Piedmont of central Virginia.
I am not very well suited for field biology in the mountains. I learned this back in March when our Bryology class went to the Highlands Biological Station, up in the NC mountains. Climbing uphill was tough on me, and to try and keep up I would hunch over and hurry upwards, which ruined my back for a few days afterwards. Most of it is because I'm out of shape, and I'm working on that. The MLBS, meanwhile, is at 3800 feet, and these "Bear Cliffs" were at 4100 feet, so there was a bit of a climb to come for me. My back and legs screaming, I eventually made it up to the top, about 15 minutes after everyone else:

My GPS disagreed with the elevation listed on the sign, but that wasn't a primary concern. This picture was taken across a crevice that had a sheer drop of 100 feet or so, and I had to negotiate around to finally get to the flat-topped rock where everyone was sitting. I said hello and then wanted to take in the view, which was striking:

I then sat down on the rock to enjoy the view (and catch my breath), and I leaned back. One hand found rock behind me, but the other went into something moist and squishy. Alarmed, I spun around and let out a high pitched "Cool!" that caused the others to look at me very weirdly. Here's what I saw:

Funaria! On the top of this mountain! I whipped out my collecting bags and my knife and carefully sampled the population. The trip up the mountain was definitely worth it. When I got back to Duke, I was telling my advisor about this and his colleague across the hall declared afterwards: "A success, of Accidental Bryology!" Indeed, it was.
Since I'm taking a Bryology class this semester, I've gotten in the habit of taking my collecting bag wherever I go. It's complete with my GPS, Camera, small paper bags, and pens. It also contains a 14x hand lens, which others have lovingly referred to as a "nerd beacon," as you can always tell a naturalist from the small lens draped around his neck. Throughout the day, I kept looking for Funaria in its customary places: near old campfires, along the edges of the paths. Plenty of moss everywhere, but no Funaria. That was fine, and I had fun telling the laboratory-stricken among us all about bryophytes. Later in the day, there was a movement to hike up to Bear Cliffs, which is a sheer cliff-face that overlooks all of the Piedmont of central Virginia.
I am not very well suited for field biology in the mountains. I learned this back in March when our Bryology class went to the Highlands Biological Station, up in the NC mountains. Climbing uphill was tough on me, and to try and keep up I would hunch over and hurry upwards, which ruined my back for a few days afterwards. Most of it is because I'm out of shape, and I'm working on that. The MLBS, meanwhile, is at 3800 feet, and these "Bear Cliffs" were at 4100 feet, so there was a bit of a climb to come for me. My back and legs screaming, I eventually made it up to the top, about 15 minutes after everyone else:
My GPS disagreed with the elevation listed on the sign, but that wasn't a primary concern. This picture was taken across a crevice that had a sheer drop of 100 feet or so, and I had to negotiate around to finally get to the flat-topped rock where everyone was sitting. I said hello and then wanted to take in the view, which was striking:
I then sat down on the rock to enjoy the view (and catch my breath), and I leaned back. One hand found rock behind me, but the other went into something moist and squishy. Alarmed, I spun around and let out a high pitched "Cool!" that caused the others to look at me very weirdly. Here's what I saw:
Funaria! On the top of this mountain! I whipped out my collecting bags and my knife and carefully sampled the population. The trip up the mountain was definitely worth it. When I got back to Duke, I was telling my advisor about this and his colleague across the hall declared afterwards: "A success, of Accidental Bryology!" Indeed, it was.
Friday, April 25, 2008
Matt's Moss Work: A Primer
Welcome to Gametophyte Junction. Here, I will be making (hopefully) frequent updates on my progress through grad school. Most who know me, visiting this site, already have a very vague idea of what I'm doing: evolutionary biology of mosses. This site is intended to give a more in-depth view of what the crap I'm spending my mid-twenties doing. It's also a place for me to practice boiling down my research to layman terms, and hopefully also a place for me to come back to in a few years to observe how wide-eyed I was as a first-year grad student. So, feel free to say hi in the comments, and please: ask questions! If you're interested, then I'm doing a good job making it sound interesting, and that would make me happy. Anyway, I thought I'd start with a short overview of what I'm working on and the kinds of questions I'm interested in. I'll probably go into further detail on lots of these things in future posts, so stay tuned!
The genus of moss I'm going to be working on is called Funaria, which looks like this:
It's a very common, small, weedy type moss that grows in all kinds of disturbed habitats: recently burned forests, roadsides, sidewalk cracks, etc. It is a monoicious moss, which means it has produces both male and female parts, eventually releasing egg and sperm from the same plant. I'll try to avoid jargon as much as possible, and so I'll refer to this condition as being "bisexual." It will probably get me blocked by filters, but that's okay, it keeps the riffraff out. It's also very closely related to the species Physcomitrella patens, which recently became the first moss to have its entire genome sequenced. That's going to make a lot of what I'll be doing a lot easier.
To explain what it is I'll be doing with this moss, you'll have to take a trip with me back to basic bio:
Most organisms are diploid; they have two copies of every chromosome for most of their life cycle- this is true of all animals and most all plants. The organism you see (whether it's a dog or a tree) is diploid. All organisms, when they sexually reproduce, reduce their chromosome number to "haploid" to make sperm and egg, which then fuse to produce a new diploid individual. In plants, the haploid stage lasts a bit longer, with multicellular haploid structures forming from which the sperm and egg are derived. This is called an "alternation of generations," and in mosses, it is to the extreme. In the Funaria picture, the green tufts at ground level are the haploid stage- a bisexual "gametophyte" that produces both sperm and egg
(gametes). These fuse just like in any organism and produce a diploid individual- in the picture, that's the green glob on the end of a long white stem. Inside the green glob, meiosis occurs, producing haploid spores that are released to grow into new gametophytes.
Ok, so a couple of interesting things going on here, one of which is the issue of inbreeding. One of the fundamental rules of evolutionary biology is that inbreeding is bad... very bad. Recently,
the entire diploid genome of DNA-helix discoverer James Watson was sequenced, and he was found to have 12 lethal mutations... but he only had one copy of those mutations, and because they were recessive (like blue eyes), he is able to survive. But he most likely shares many of
these 12 mutations with his sister, and if they were to inbreed, the child would not survive, because the chances are very high that the child would have two copies of a lethal mutation. In evolutionary terms, this decreased "fitness" due to inbreeding is known as "inbreeding depression," and is the measure of decreased fitness that is caused by related individuals mating.
So, back to the moss. Remember I said that Funaria is bisexual, and produces both egg and sperm on the same plant- these egg and sperm will be (genetically) completely identical to one another, and if they fuse to produce a diploid, it will immediately have two identical copies of every gene, including any recessive mutations. This could be very bad news for the diploid offspring, if the mutations are lethal or even simply disadvantageous. So, one of the basic questions I want to ask is what relationship is there between relatedness and fitness in a moss. I'll be growing moss from different populations together to see if there is an effect- do related individuals produce unfit offspring, and do unrelated individuals produce more fit offspring? I'll be measuring relatedness by using a DNA-fingerprinting technique known as microsatellites- short regions of DNA repeat the same thing over and over, and it varies from individual to individual how many copies there are. By comparing the patterns of the numbers of copies in the offspring, I can estimate how related its parents were.
The other major part of my research involves the components of mating success. Many studies have been done looking at this topic in other organisms, under the umbrella of sexual selection. Briefly, this kind of selection typically involves animal species which evolve specialized characters that are most related to finding mates. For example, deer bull antlers are characteristics by which does select their mate- the bigger the antler, the higher probability of mating. Similarly, cardinals which are bright red have a better chance at mating. There are a few hypotheses regarding why such traits evolve, but I'm more interested in which traits are important, rather than why. When we see a patch of moss like pictured above, what traits of a particular individual give it a higher chance of mating? I will also be testing hypotheses with my mosses using a combination of field research and experimental studies, such as having two male mosses compete over a single female, and measuring the offspring to determine who got the girl.
Well, I think that's a good start. Stay tuned for more hot moss on moss action! I mean, serious science.
The genus of moss I'm going to be working on is called Funaria, which looks like this:
It's a very common, small, weedy type moss that grows in all kinds of disturbed habitats: recently burned forests, roadsides, sidewalk cracks, etc. It is a monoicious moss, which means it has produces both male and female parts, eventually releasing egg and sperm from the same plant. I'll try to avoid jargon as much as possible, and so I'll refer to this condition as being "bisexual." It will probably get me blocked by filters, but that's okay, it keeps the riffraff out. It's also very closely related to the species Physcomitrella patens, which recently became the first moss to have its entire genome sequenced. That's going to make a lot of what I'll be doing a lot easier.
To explain what it is I'll be doing with this moss, you'll have to take a trip with me back to basic bio:
Most organisms are diploid; they have two copies of every chromosome for most of their life cycle- this is true of all animals and most all plants. The organism you see (whether it's a dog or a tree) is diploid. All organisms, when they sexually reproduce, reduce their chromosome number to "haploid" to make sperm and egg, which then fuse to produce a new diploid individual. In plants, the haploid stage lasts a bit longer, with multicellular haploid structures forming from which the sperm and egg are derived. This is called an "alternation of generations," and in mosses, it is to the extreme. In the Funaria picture, the green tufts at ground level are the haploid stage- a bisexual "gametophyte" that produces both sperm and egg
(gametes). These fuse just like in any organism and produce a diploid individual- in the picture, that's the green glob on the end of a long white stem. Inside the green glob, meiosis occurs, producing haploid spores that are released to grow into new gametophytes.
Ok, so a couple of interesting things going on here, one of which is the issue of inbreeding. One of the fundamental rules of evolutionary biology is that inbreeding is bad... very bad. Recently,
the entire diploid genome of DNA-helix discoverer James Watson was sequenced, and he was found to have 12 lethal mutations... but he only had one copy of those mutations, and because they were recessive (like blue eyes), he is able to survive. But he most likely shares many of
these 12 mutations with his sister, and if they were to inbreed, the child would not survive, because the chances are very high that the child would have two copies of a lethal mutation. In evolutionary terms, this decreased "fitness" due to inbreeding is known as "inbreeding depression," and is the measure of decreased fitness that is caused by related individuals mating.
So, back to the moss. Remember I said that Funaria is bisexual, and produces both egg and sperm on the same plant- these egg and sperm will be (genetically) completely identical to one another, and if they fuse to produce a diploid, it will immediately have two identical copies of every gene, including any recessive mutations. This could be very bad news for the diploid offspring, if the mutations are lethal or even simply disadvantageous. So, one of the basic questions I want to ask is what relationship is there between relatedness and fitness in a moss. I'll be growing moss from different populations together to see if there is an effect- do related individuals produce unfit offspring, and do unrelated individuals produce more fit offspring? I'll be measuring relatedness by using a DNA-fingerprinting technique known as microsatellites- short regions of DNA repeat the same thing over and over, and it varies from individual to individual how many copies there are. By comparing the patterns of the numbers of copies in the offspring, I can estimate how related its parents were.
The other major part of my research involves the components of mating success. Many studies have been done looking at this topic in other organisms, under the umbrella of sexual selection. Briefly, this kind of selection typically involves animal species which evolve specialized characters that are most related to finding mates. For example, deer bull antlers are characteristics by which does select their mate- the bigger the antler, the higher probability of mating. Similarly, cardinals which are bright red have a better chance at mating. There are a few hypotheses regarding why such traits evolve, but I'm more interested in which traits are important, rather than why. When we see a patch of moss like pictured above, what traits of a particular individual give it a higher chance of mating? I will also be testing hypotheses with my mosses using a combination of field research and experimental studies, such as having two male mosses compete over a single female, and measuring the offspring to determine who got the girl.
Well, I think that's a good start. Stay tuned for more hot moss on moss action! I mean, serious science.
Subscribe to:
Comments (Atom)
